Coral Springs, FL – March 22, 2017 – DB Surgical, a business specializing in the distribution of surgical instruments and technology, today announced it has signed an exclusive distribution agreement with BOSS Instruments, Ltd. that expands on its current coverage of Florida and Alabama, to include much of the state of Georgia.
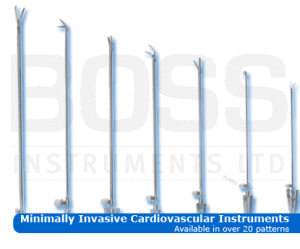
Under the terms of the agreement, DB Surgical, which has developed into one of the top BOSS distributors in the United States over the last ten years, has been granted exclusive rights to distribute BOSS Instruments complete line of specialty instruments and retractor systems throughout Florida, Alabama and most of Georgia. With the BOSS Instruments portfolio consisting of more than 25,000 patterns of specialty surgical instruments and retractors, it offers an ideal complement to DB Surgical’s other products, such as surgical microscopes, electrosurgical products, equipment drapes and surgical headlights. The combination of surgical solutions from DB Surgical and BOSS Instruments allows DB Surgical to provide customers a complete range of surgical equipment.
About DB Surgical: DB Surgical is dedicated to bringing transformational surgical technologies to hospitals and surgery centers. The company focuses on introducing tools that enable surgeons to advance patient outcomes beyond those available today. Since 1997, DB Surgical has worked with industry-leading surgeons to understand and meet the evolving needs of healthcare providers. The company is based in Coral Springs, FL and has 19 professionals located throughout Florida, Georgia, and Alabama focusing on the Neuro, Spine, ENT, Cardiovascular, Ophthalmic, and Plastic Reconstructive surgical disciplines. In addition to providing cutting-edge technologies, DB Surgical also offers a complete line of specialty surgical products designed to create value within the healthcare supply chain. More information is available at www.DBSurgical.com.
Contact Information: Phone: 954.340.2727 Fax: 954.340.1221 Email: [email protected]
About BOSS Instruments: BOSS Instruments is a surgical instrument company which concentrates on the manufacture and continual development of specialty lines of instruments in the following areas: Ear, Nose, and Throat, General Surgery, Laparoscopy, Neurosurgery, Obstetric/Gynecology, Ophthalmic, Orthopedic, Plastic, Table Mounted Retractors, Vascular/Cardiovascular, and Bariatric. More information is available at www.BOSSinst.com.
Contact Information: Phone: 800.210.2677 Fax: 540.832.5515 Email: [email protected]